Introduction to Dalton’s atomic theory
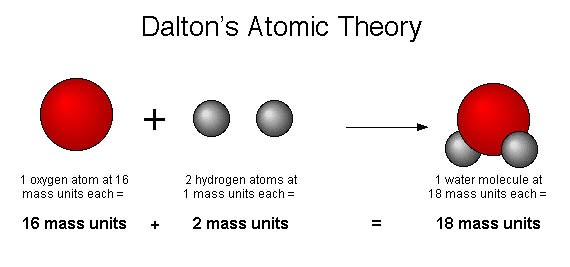
The theory that all matter is made up of very tiny indivisible particles is called atomic theory of matter. Dalton put forward his atomic theory of matter in 1808. The various postulates of Dalton’s atomic theory of matter are as follows:-
- All the matter is made up of very small particles called ‘atoms’.
- Atoms cannot be divided.
- Atoms can neither be created nor destroyed.
- Atoms are of various kinds. There are as many kinds of atoms as are elements.
- All the atoms of a given element are identical in every respect, having the same mass, size and chemical properties.
- Atoms of different elements differ in mass, size and chemical properties.
- Chemical combination between two (or more) elements consists in the joining together of atoms of these elements to form moecules of compounds.
- The ‘number’ and ‘kind’ of atoms in a given compund is fixed.
- During chemical combination, atoms of different elements combine in small whole numbers to from compounds.
- Atoms of the same elements can combine in more than one ratio to form more than one compound.

John Dalton
For elements that combined in multiple ratios, their combinations were assumed to be the simplest ones possible. Two combinations resulted in a binary and a ternary compound.This was merely an assumption, derived from faith in the simplicity of nature. No evidence was then available to scientists to deduce how many atoms of each element combine to form compound molecules. But this or some other such rule was absolutely necessary to any incipient theory, since one needed an assumed molecular formula in order to calculate relative atomic weights. In any case, Dalton’s “rule of greatest simplicity” caused him to assume that the formula for water was OH and ammonia was NH, quite different from our modern understanding (H2O, NH3).
Despite the uncertainty at the heart of Dalton’s atomic theory, the principles of the theory survived. To be sure, the conviction that atoms cannot be subdivided, created, or destroyed into smaller particles when they are combined, separated, or rearranged in chemical reactions is inconsistent with the existence of nuclear fusion and nuclear fission, but such processes are nuclear reactions and not chemical reactions. In addition, the idea that all atoms of a given element are identical in their physical and chemical properties is not precisely true, as we now know that different isotopes of an element have slightly varying weights. However, Dalton had created a theory of immense power and importance. Indeed, Dalton’s innovation was fully as important for the future of the science as Antoine Laurent Lavoisier’s oxygen-based chemistry had been.
Contents –
- Introduction To Dalton’s atomic theory
- Explained in Detailed: Dalton’s atomic theory
- Part 1: All matter is made of atoms
- Part 2: All atoms of a given element are identical in mass and properties.
- Part 3: Compounds are combinations of two or more different types of atoms.
- Part 4: A chemical reaction is a rearrangement of atoms.
- What have we learned since Dalton proposed his theory?
- Drawbacks of Dalton’s atomic theory
Explained In Detailed: Dalton’s atomic theory
Part 1: All matter is made of atoms.
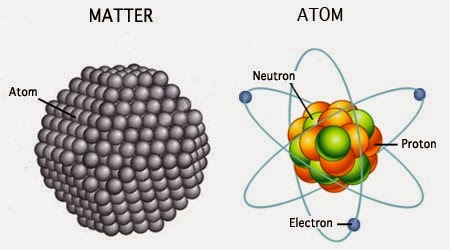
A magnified figure of matter and atom
It is important to note that since Dalton did not have the necessary instruments to see or otherwise experiment on individual atoms, he did not have any insight into whether they might have any internal structure. We might visualize Dalton’s atom as a piece in a molecular modeling kit, where different elements are spheres of different sizes and colors. While this is a handy model for some applications, we now know that atoms are far from being solid spheres.
Part 2: All atoms of a given element are identical in mass and properties.
Dalton proposed that every single atom of an element, such as gold, is the same as every other atom of that element. He also noted that the atoms of one element differ from the atoms of all other elements. Today, we still know this to be mostly true. A sodium atom is different from a carbon atom. Elements may share some similar boiling points, melting points, and electronegativities, but no two elements have the same exact set of properties.
In the third part of Dalton’s atomic theory, he proposed that compounds are combinations of two or more different types of atoms. An example of such a compound is table salt. Table salt is a combination of two separate elements with unique physical and chemical properties. The first, sodium, is a highly reactive metal. The second, chlorine, is a toxic gas. When they react, the atoms combine in a 1:1 ratio to form white crystals of NaCl, which we can sprinkle on our food.
Part 4: A chemical reaction is a rearrangement of atoms.
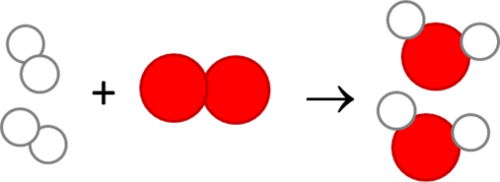
The diagram below represents another chemical reaction. We have oxygen (red molecules) reacting with hydrogen (white molecules) to produce water.
In the fourth and final part of Dalton’s atomic theory, he suggested that chemical reactions don’t destroy or create atoms. They merely rearranged the atoms. Using our salt example again, when sodium combines with chlorine to make salt, both the sodium and chlorine atoms still exist. They simply rearrange to form a new compound.
What have we learned since Dalton proposed his theory?
Despite these caveats, Dalton’s atomic theory is still mostly true, and it forms the framework of modern chemistry. Scientists have even developed the technology to see the world on an atomic level!
Drawbacks of Dalton’s atomic theory
Some molecules of different gases
It is now known that some of the statements of Dalton’s atomic theory of matter are not exactly correct.Some of the drawbacks of the Dalton’s atomic theory of matter are given below:
- One of the major drawbacks of Dalton’s atomic theory of matter is that atoms were thought to be indivisible (which cannot be divided). We now know there under special circumstances, atoms can be further divided into still smaller particles called electrons, protons and neutrons. So atoms are themselves made up of three particles: electrons, protons and neutrons.
- Dalton’s atomic theory says that all the atoms of an element have exactly the same mass.It, is however, now known that atoms of the same element can have slightly different masses.
- Dalton’s atomic theory said that atoms of different elements have different masses. It, is however, now known that even atoms of different elements can have the same mass.